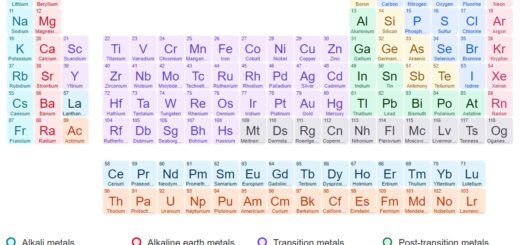
What is an Atom
Pic Credit : Wikipedia
Atoms are the smallest units of matter that retain the properties of an element. They consist of three basic components: protons, neutrons, and electrons.
Protons are positively charged particles that are found in the nucleus, or center, of an atom. The number of protons in an atom determines the element it belongs to and is referred to as the atomic number.
Neutrons, on the other hand, are neutral particles that also reside in the nucleus. They help to hold the protons together in the nucleus by providing a strong force. The number of neutrons in an atom can vary, and this variation gives rise to isotopes of the same element.
Electrons are negatively charged particles that orbit the nucleus in shells. The electrons are much lighter than the protons and neutrons and occupy the outermost regions of the atom. The number of electrons in an atom is equal to the number of protons, which ensures that the atom is electrically neutral.
Atoms can bond together to form molecules, which are the building blocks of all matter. The type of bond between atoms depends on the number of electrons they share or transfer. Ionic bonds occur when electrons are transferred from one atom to another, while covalent bonds occur when electrons are shared between two atoms.
The study of atoms and their properties is known as atomic physics. This field of study has led to a number of important discoveries and technological advancements, including the development of atomic energy and the use of atomic clocks for precise timekeeping.
In conclusion, atoms are the fundamental building blocks of matter and play a crucial role in our understanding of the world around us. From the study of atomic physics, we have gained valuable knowledge and technological advancements that have greatly impacted society.
#CHATGPT generated content