ALLOTROPY of OXYGEN
Summary of Allotropy of oxygen
Solved exercise of Allotropy of oxygen

Summary
Oxygen is critical to human life and that going without it for more than just a couple of minutes is incompatible with maintaining that life. And if you’ve had a look around the medical, industrial and aerospace worlds, you’ve probably seen oxygen storage systems well represented in each of these areas.The air which we breathe in consists of 21% oxygen by volume. The air which we breathe out consists of 16% oxygen by volume. This shows that respiration makes use of oxygen.
Why do the astronauts carry cylinders containing oxygenated air?

As we go up in the space, the oxygen available for breathing becomes less and above certain height in space there is no oxygen at all, so the astronauts carry oxygen cylinders with them to ensure sufficient oxygen supply for breathing.
The life exists on the earth because of the presence of oxygen in the atmosphere. Oxygen exists in two allotropic forms.They are
1. Diatomic molecule (O2)
- Triatomic molecule (O3) which is called ozone.
Diatomic molecule (O2)
The oxygen present around us is in the form of diatomic molecule. It is colourless, odourless and tasteless gaseous substance. The air in the troposphere consists of nearly 21% by volume of O2 molecules.
- Oxygen exists in two allotropic forms. They are diatomic molecule and triatomic molecule (ozone).
- Oxygen is colorless, odorless and tasteless gaseous substance found in the troposphere.
- Oxygen is chemically very reactive.
- Ozone is found at about 16 km height from the earth’s surface. Ozone prevents the high energy U.V rays to reach the earth.
Properties of oxygen
- Oxygen reacts with other elements to give the respective oxides
- Magnesium + Oxygen → Magnesium oxide
- It reacts with some compounds and decomposes them
- Mercuric Sulphide + Oxygen → Mercury + Sulphur dioxide.
Triatomic Molecule (O3) :
- Oxygen reacts with other elements to give the respective oxides
- Magnesium + Oxygen → Magnesium oxide
- It reacts with some compounds and decomposes them
- Mercuric Sulphide + Oxygen → Mercury + Sulphur dioxide.
Properties of Triatomic Molecule (O3) :
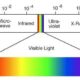
Sun emits radiations, among which some are harmful to the living organisms.
But all the radiations do not reach the earth, as the earth is protected by a blanket of gas.
Ozone is allotropic form of oxygen. It is a triatomic molecule.
Ozone is a light bluish gas found in the upper layer of the atmosphere (stratosphere
It prevents ultraviolet rays coming from the sun to reach the earth
Thus it protects the life on the earth from the harmful effects of U.V rays,
Which can cause skin cancer and destroy many organic species etc.
Depletion of the ozone layer

The decrease in the quantity ozone depleted region Fig. 11.2 of ozone in the upper layer of the atmosphere is called depletion of ozone.
Pollutants like oxides of nitrogen and chlorofluorocarbon react with ozone molecules and destroy them.
Properties of Ozone
- Ozone is a poisonous gas with fishy smell.
- It causes respiratory problems, if inhaled
- It damages plants and trees, if comes in contact with them
- It causes damages to automobile tyres and asphalt, if comes in contact with them.
Exercises
I. Answer the following questions
1. Name the allotropic forms of oxygen.
Ans:Is generally known as oxygen, but may be called di oxygen, di atomic oxygen,molecular oxygen, or oxygen gas to distinguish it from the element itself.oxygen is most commonly encountered in this form.
2. What are the properties of oxygen?
. What are the properties of oxygen?
Ans the properties of oxygen are
- It is tasteless and colorless gas
- It is in gaseous form at room temperature
- It is heavier than air
- It reacts with metals to form basic oxides
- It reacts with non-metals to form acidic oxides.
3. What is ozone? List out its properties.
Ans:The ozone layer is a deep layer in the stratosphere, encircling the Earth, that has large amounts of ozone in it.
Properties of Ozone are
- Ozone Absorbs radiation strongly in the ultraviolet region of the atmospheric spectrum
- ozone is a blue gas, with a strong irritating smell.
- protects the Earth and its inhabitants from the harmful ultraviolet radiation of the Sun.
4. How does ozone layer protect the living organisms on the earth?
Ans: Sun gives out ultraviolet radiations that are extremely harmful to human. The ozone layer will work out as a wavelength that can protect the life of every organism. This decreases the risk of life towards the organism since they will not have any reaction of the rays.
5. What is depletion of ozone layer?
Ans : Ozone layer depletion is the gradual thinning of the earth’s ozone layer in the upper atmosphere caused due to the release of chemical compounds containing gaseous bromine or chlorine from industries or other human activities.
7. Suggest some measures to prevent the depletion of ozone layer
Ans: Stop using refrigerators, coolers using CFC as refrigerant.
- Decrease air pollution by planting trees.
- Decrease use of air polluting automobiles.
- Avoid use of nitrous oxide which depletes ozone.
- Decrease use of unnecessary pesticides