ALLOTROPY OF CARBON
Summary of allotropy of carbon
Solved exercise of allotropy of carbon
Summary
Carbon with atomic number 6 and represented by the symbol ‘C’ in the periodic table is one of the most influential elements we see around us.carbon is one of the elements which shows allotrop.
Crystalline forms of carbon
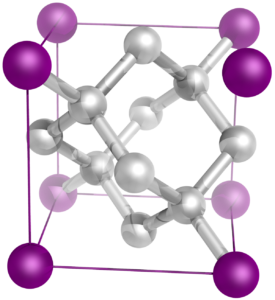
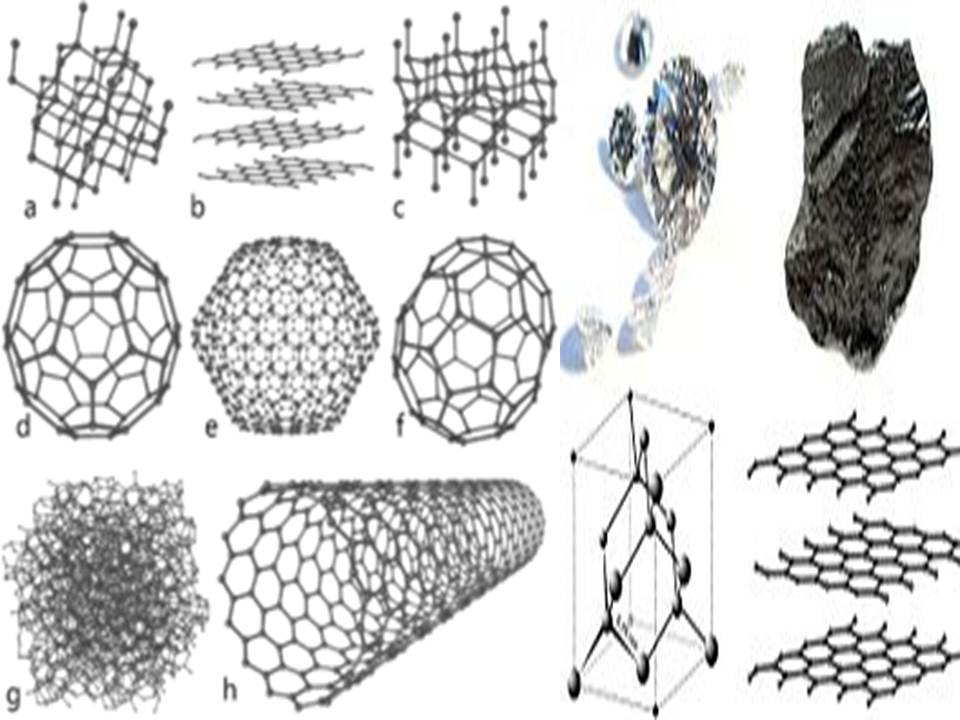
The solid in which the atoms are arranged in a definite geometrical shape is called crystalline solid. Diamond, graphite and fullerene are the crystalline allotropes of carbon.
Diamond and its Structure

Diamond is the hardest natural substance known It is found in many parts of the world such as Brazil, South Africa, Australia and United States of America. Diamond is generally colourless. However, the coloured varieties – yellow, brown red, green, blue, grey are also found in nature.It is used for cutting glasses and drilling rocks.The central carbon atom is bonded to four carbon atoms placed at the vertices of a tetrahedron The other carbon atoms in turn are also tetrahedral bonded to four atoms each.
Properties of diamond :
- Diamond is the hardest crystalline form of carbon
- It is a bad conductor of electricity and a good conductor of heat.
- Properly cut diamond reflects a great percentage of light falling on it.
- It sublimes at 3,5000 C.
- When diamond is ignited, it burns in air at 9000 C and in oxygen at 7000 C to give carbon dioxide.
Uses of diamond

- Diamond is used in jewellery.
- It is used in the tools of cutting glasses and drilling rocks
Graphite

The lead is actually not the metal lead, but graphite mixed with some clay. Graphite is soft, black and crystalline solid. It is found in large deposits in many countries like India, China and South Korea. While writing with a pencil what happens if you apply more force It breaks.
Uses of graphite

- Graphite is mixed with a very little amount of clay and used as pencil lead.
- Graphite powder is mixed with oil and used as lubricant for machines
- This lubricant is also used in the machines which work at high temperature.
Non – Crystalline or Amorphous forms of carbon
Arrangement of atoms in amorphous solids is not regular. The amorphous forms of carbon are charcoal, coal, coke, gas carbon and lampblack (soot). Among these, except coal, all others are man made.
Charcoal

Charcoal is prepared by the process of destructive distillation of wood.
Wood charcoal is prepared by the destructive distillation of wood.
wood gas. Wood gas is combustible. The main constituent of wood gas is methane (CH4).
Uses of charcoal

- Charcoal is used as a fuel
- Charcoal is used in the manufacture of gun powder
- charcoal is used in Gas mask used in the gas masks and in purification of water
- Bone charcoal is used in sugar industry to decolourise sugar
- Sugar charcoal is used to obtain metals from their oxides
Coal and its uses
- Coal is a natural resource and an allotropic form of carbon.
- Coal is used as fuel in thermal power plants and in steam engines
- It is used in the manufacture of coal gas, coal tar and coke.
- Coal is used in the manufacture of fuel gases like water gas and producer gas.
Coke

Coke is the purest form of carbon. It burns without smoke. It is a good conductor of electricity.
Uses of coke

- Coke is used as a fuel,
- It is used in the extraction of iron from its ore.
Gas Carbon
When coal is destructively distilled the deposit formed on the sides and roofs of retorts are called gas carbon.
Lampblack
Lampblack is soft black powder obtained by burning carbon rich substances such as turpentine oil or other vegetable oil in an insufficient supply of air.
Uses of lampblack
- Lampblack is used in the manufacture of printing ink, black paint, Indian ink and shoe polish.
- It is used as filler for rubber in making tyres and plastics.
Compounds of carbon
Carbonates and bicarbonates
- The rava idlis, vadas and dosas prepared in the hotels are softer than those prepared at home.
- They mix a small quantity of a white powder just before cooking. It is called baking powder
- It consists of sodium bicarbonate
- For washing of clothes they use white crystals It is called washing soda
- some of the carbonates and bicarbonates which are more useful in our daily life.
Calcium carbonate (CaCO3)
Many natural things like limestone, shells, egg shells, conch and marble consist of calcium carbonate
- Calcium carbonate is formed by the reaction of carbonic acid with calcium hydroxide.
- Carbonic acid + Calcium hydroxide → Calcium carbonate + Water.
- Lime water consists of calcium hydroxide. The blown air consists of carbon dioxide.
- Carbon dioxide reacts with calcium hydroxide to form insoluble calcium carbonate. So, the mixture becomes milky white
- Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water.
Uses of calcium carbonate
Calcium carbonate is used in the manufacture of cement and glass.
It is used in the extraction of iron from its ore in order to remove the earthy impurities.
Sodium carbonate [Na2CO3.10 H2O]
Sodium carbonate which is available in the powdered form is called soda ash. The crystalline form is called washing soda. It is also called sodium carbonate decahydrate.
Carbonic acid + Sodium hydroxide → Sodium carbonate + Water
Sodium bicarbonate (NaHCO3):
Sodium bicarbonate is called baking soda. When carbonic acid is treated with limited quantity of sodium hydroxide, sodium bicarbonate is formed.
Carbonic acid + Sodium hydroxide → Sodium bicarbonate (limited) + Water
Exercises
I.Four alternatives are given under each complete/ incomplete statement. Choose the correct answer and put a tick (P) mark against it :
1. The structure of diamond is
a) hexagonal b) tetrahedral
c) soccer ball d) amorphous
Ans : b) tetrahedral
2.Diamond is used as a gem stone, because it
a) does not possess free electrons
b) is a giant crystal
c) possesses covalent bonds
d) bends a great percentage of light
Ans: d) bends a great percentage of light
3. Graphite is used as electrodes in dry cells, because it is
a) smooth and slippery
b) a good conductor of electricity
c) heat resistant
d) a good moderator
Ans: b) a good conductor of electricity
4. Coke is a good fuel, because it
a) conducts electricity
b) reacts with metallic oxides
c) burns without smoke
d) is amorphous form of carbon.
Ans : c) burns without smoke
5. The allotrope of carbon consisting of discrete molecules is
a) diamond b) graphite
c) fullerene d) coal
Ans: fullerene
6. Substances obtained when coal is subjected to destructive distillation are
a) coal tar and coke b) coal tar and fullerene
c) coke and charcoal d) coal tar and lamp black
Ans: coal tar and coke
7.Charcoal floats on water, because it is
a) denser than water b) soft
c) porous d) opaque
Ans: a) denser than water
8. Allotropy is caused due to
a) arrangement of electrons b) arrangement of atoms
c) components of atoms d) components of nucleus
Ans: porous
9. The gas which turns lime water milky is
a) sulphur dioxide b) nitrogen dioxide
c) chlorine d) carbon dioxide
Ans: carbon dioxide
10. The compound of carbon which is used in the manufacture of cement is
a) calcium carbonate b) sodium carbonate
c) sodium bicarbonate d) potassium carbonate
Ans: calcium carbonate
11. The chemical name of baking soda is
a) sodium carbonate b) sodium hydroxide
c) sodium bicarbonate d) sodium sulphate
Ans: sodium bicarbonate
Match the following with answers
| 1. | bone charcoal | Decolourization of sugar. |
| 2. | . gas carbon | the deposits formed on the roofs of the retorts during destructive distillation of coal |
| 3 | Diamond | Glass cutting instrument |
| 4 | fullerene | yellow spherical ball |
| 5 | coal | Formed by slow decomposition of plants |
- State whether the following statements true or false.If false give reasons
- Coke reacts with metal oxides to give metals. ( True)
- Fullerenes cannot be used as lubricants. (False )
- Coal gas is obtained when coal is subjected to destructive distillation. ( True)
- Sugar charcoal consists of a lot of carbon compounds as impurities. ( False)
- Gas carbon is a bad conductor of electricity. ( False )
Answer the following questions
1.What is allotropy? Name the allotropic forms of carbon. Mention one use of each.
Ans : Allotropy is a single element exist in two or more forms ,in the same physical state carbon has following allotropes.
graphite: used as lubricants in machines where high temperatures are generated
diamond: used as lubricants in machines where high temperatures are generated.
2.Explain the preparation of wood charcoal, with help of a neat labelled diagram

Ans: The wood is heated in the absence of oxygen and the product derived is wood charcoal. The main advantage of using wood charcoal instead of wood is the removal of water from wood during the process of pyrolysis.
3.What are the uses of charcoal?
Ans: Charcoal has been used since earliest times for a large range of purposes including art and medicine, but by far its most important use has been as a metallurgical fuel.
Charcoal is the traditional fuel of a blacksmith’s forge and other applications where an intense heat is required.
4.What are the uses of coal?
Ans : The uses of coal are in electricity generation, steel production, cement manufacturing and as a liquid fuel. Different types of coal have different uses. Steam coal also known as thermal coal – is mainly used in power generation.
5.Which allotrope of carbon is used in the manufacture of black paint?
Ans : Lamp black is a velvety black powder. It is used in the manufacture of Indian ink, printer’s ink, carbon papers, black paint and varnishes.
6.How is coke obtained?
Ans :Coke is a grey, hard, and porous fuel with a high carbon content and few impurities, made by heating coal or oil in the absence of air—a destructive distillation process. … A similar product called petroleum coke, or pet coke, is obtained from crude oil in oil refineries.
7.State any two uses of fullerene.
Ans : Fullerenes have been used as a carrier for gene and drug delivery systems. Also they are used for serum protein profiling as meldi material for biomarker discovery.
8.How is calcium carbonate prepared in the laboratory?
Ans : calcium carbonate is obtained by using carbon dioxide and slaked lime as raw materials. When carbon dioxide is passed through slaked lime, calcite is obtained. … However, if carbon dioxide is passed in excess, it forms the soluble calcium hydrogen-carbonate.
9. Write any one use of the following
- Calcium carbonate: It is a white insoluble powder-like substance which occurs naturally in minerals, chalk marble, limestone, calcite, shells, pearl, etc. It is also used as fillers in cosmetics
- . Sodium carbonate: It is used in the manufacture of detergents, soaps, paper. Also used in the manufacture of water glass borax, sodium phosphate, and many other sodium compounds.
- Sodium bicarbonate: Sodium bicarbonate reduces stomach acid. It is used as an antacid to treat heartburn, indigestion, and upset stomach.