Chemical Bonding

Summary
Every substance in the universe has the tendency to attain stability.Elements like Helium,
Neon, Argon, Krypton, Xenon and Radon are stable elements.
Noble Gases

These stable elements have either two or eight electrons in their outermost shells. These stable elements are called noble gases or inert gases. And they are non reative.They are also called rare gases.
Some of the noble gases are Helium,Neon,Argon,Krypton,Xenon,Radon.
Electronic configuration of noble gases

The formation of molecules of compounds and elements depend on several factors. One of the most important factors is its electronic configuration.
Elements that have an electronic configuration other than
that of the noble gases try to attain stability either by,
- Transfer of electrons (by donating or gaining electrons) from other atoms.
- Sharing of electrons with other atoms.
Its electronic configuration is 2,8,1. One electron in sodium’s outer most shell makes it
unstable. Its tendency is to attain stability. It can do so by combining with chlorine whose electronic configuration is 2,8,7 forming the compound sodium chloride.
Chemical bond.
A chemical bond is defined as a mutual attraction or link between two atoms resulting from a redistribution of the electrons in their outer most shell.
The bond formed in the sodium chloride molecule is by the transfer of one electron from sodium atom to the chlorine atom. Chemical bonds can also be formed other wise.
Types of chemical bonds :
There are two types of chemical bonds.
- Electrovalent bond or ionic bond.
- Covalent bond.
Electrovalent bond or Ionic bond:
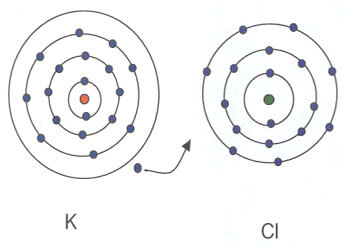
In the formation of the compound potassium chloride, one electron in the outer most shell of potassium is transferred to the outer most shell of chlorine which has 7 electrons.
From the above example
In the above examples of sodium chloride, potassium chloride and magnesium oxide, the link between the atoms is formed by complete transfer of one or two electrons.
The chemical bond formed as a result of the transfer of electrons from on atom of an element to an atom of another element is called an electrovalent bond or an ionic bond. Compounds formed by electrovalent or ionic bonds are called electrovalent or ionic compounds.
Covalent bonds

The atomic number of hydrogen is 1. That means its one and only outer most shell contains one electron. To attain stability it needs 1 electron to complete its shell.
This molecule of hydrogen is represented as H _ H. ‘ _ ’ indicates a pair of electrons being shared. Thus hydrogen molecule is formed by the sharing of one pair of electrons. This is a single
covalent bond.Electronic configuration of chlorine is 2,8,7. A chlorine molecule is formed by the sharing of one pair of electrons. It is a single covalent bond.
Compound formed by a shared pair of electrons.

Hydrogen and chlorine atoms share one pair of electrons forming a hydrogen chloride molecule. The hydrogen chloride molecule is formed by a single covalent bond.
Covalent bond or molecular bond
The chemical bond formed between two or more combining atoms by mutual sharing of one or more electrons of non- metallic atoms is called a covalent bond or molecular
bond.The molecule or compound formed due to covalent bonds is called a covalent molecule or covalent compound.
Ionic compounds and covalent compounds have specific characteristics.
| Sl. No. | Property | Ionic compounds | Covalent compound |
| 1 | Structure | Crystalline, hard and brittle at room temperature | Soft solids or liquids or gases at room temperature |
| 2 | Conductivity | Good conductors of electricity in molten state | Generally bad conductors of electricity. (except graphite). |
| 3 | Melting and boiling point High melting point and boiling point. Low melting point and boiling point. | High melting point and boiling point | . Low melting point and boiling point. |
| 4 | Solubility | Soluble in water but insoluble in organic solvents like benzene. | soluble in organic solvents |
| 5 | Density | High density | Low density. |
| 6 | Stability | Very stable. | Very unstable. |
Exercise
I. Four alternatives are given under each complete/incomplete statement. Choose the correct answer and put a tick () mark against it :
- Single shell atoms try to acquire this configuration
a) octet b) duplet
c) triplet d) none of the given
Ans:b) duplet
- On combining with other elements chlorine acquires
the configuration of
a) argon b) helium
c) neon d) krypton
Ans:a) argon
- It is a noble gas
a) chlorine b) calcium
c) radon d) oxygen
Ans:c) radon
- Noble gases are non reactive because of
a) duplet configuration
b) octet configuration
c) duplet and octet configuration
d) duplet or octet configuration
Ans:d) duplet or octet configuration
II. Fill in the blanks with suitable words :
- The electronic configuration of sodium is (Ne) 3s¹
- The chemical bond in potassium chloride molecule is due Lone pair to of electrons.
- Ionic compounds are poor conductors of electricity in the Solid state.
- Covalent compounds are also called molecular compounds.
- Ionic bonds are also called electrovalent bonds
VI. Answer the following questions :
1.How do elements that are not stable try to attain stability?
Ans: Elements that are not stable try to attain stability by transfer or by sharing of electrons. Noble gases have either two or eight electrons in their outer most orbit. They are stable elements.
2.What is one of the most important factors for the formation of molecules of compounds and molecules?
Ans:The distribution of the electrons of an atom or molecule in its respective structure is known as electronic configuration.For example, for an Oxygen atom, its electronic configuration would be 2, 6.
3.Define the following :
Ans:a) chemical bond:A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
b)Ionic bond:Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions.
c) covalent bond:A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.