ELEMENTS, COMPOUNDS AND MIXTURES

Matters available in nature consist of molecules or compound molecules. When these compound molecules are subdivided, elements are obtained. The smallest unit of the element is called an atom. Depending on the atoms present in the substances, they are classified as elements, compounds, and mixtures.
Elements
Elements are made up of very small particles. These are formed by particles with the same properties. Elements cannot be subdivided chemically and cannot be synthesized by other elements. Example Oxygen – O Hydrogen – H
Some elements are naturally available whereas some other elements are artificially prepared. Elements are classified as metals and non metals.
Example : Natural element – Gold Artificial element – Plutonium.
Compounds
When two are more elements combine chemically in a specific ratio and form a substance of new property it is called a compound.
Example : Water – H2O.
Water is a compound formed by the chemical combination of Hydrogen and Oxygen in the ratio 2 :1 Molecular formula is used to represent a compound.
Example : Sugar is made up of carbon, hydrogen and oxygen. But sugar does not possess any of their properties.
Mixtures
If two or more substances (elements or compounds) are mixed together in any ratio, such that they do not undergo any chemical change, but retain their individual properties, then the resulting substance is called a mixture.
Example : Soil is a mixture of sand, clay, many types of salts and residues of plants and animals.
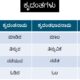
Differences between compounds and mixture Compounds Mixtures
| Compounds | Mixtures |
| 1. When two or more elements combine chemically compounds are formed | When two or more substances mix physically, mixtures are formed. |
| 2. The constituents of compounds are combined in definite ratio or proportion | The constituents of a mixture may be mixed in any proportion |
| 3. The constituent substances of a compound do not retain their original properties after combination | The constituent substances of a mixture retain their individual properties. |
| 4. The constituents of compounds cannot be separated by simple methods (without chemical reactions) | The constituents of mixtures can be separated by simple methods. |
Element, compound and mixture may be solid, liquid or gas
| Element | Compound | Mixture | |
| Solid | iron | sugar | Soil |
| Liquid | mercury | water | sea water |
| Gas | oxygen | carbon dioxide (at room temperature) | Air |
Fill in the blanks with suitable words.
1. Element consists of a group of same type of ATOM
2. Compound consists of a group of atoms of different ELEMENTS
3.A compound is When two are more elements combine chemically in a specific ratio.
4. A mixture is If two or more substances (elements or compounds) are mixed together in any ratio.
5.. Give five examples to each of these
element :- oxygen
Compound:- Water
Mixture:- Clay